To listen to the entire interview, use the player below.
AVS (Amplitude Vascular Systems) was born out of research at the University of Michigan about six years ago.
Robert Chisena, then a doctoral student in mechanical engineering at the university, and interventional cardiac specialist Hihinder Gulum, the university's chief clinical officer, developed a new method to safely break down calcium in peripheral blood vessels. We have started developing an approach.
“Very early on, when we started looking at the number of people affected by this, we knew this was a big problem, but it wasn't until we started prioritizing it above other issues that it became a big problem. “I didn't know that,' people,'' Chisena said. Medical technology insights.
AVS' balloon-based endovascular lithotripsy technology generates rapid, high-frequency, low-intensity pulses from outside the body and delivers pressure waves into blood vessels through a balloon catheter.

Robert Chisena
“The instantaneous strong impact force, and the vibrations of that instantaneous force, crack the calcium,” he explained. “If I can make an impact, [the calcium] The solids are broken down over time without damaging surrounding tissues, resulting in excellent therapeutic effects. ”
AVS' system is designed to be a minimally invasive technology that uses a single device to gently crush calcium and open arteries, requiring only a small footprint in a hospital catheterization lab. “He traverses the lesion, crushes the calcium, dilates the blood vessels, and then removes the device. All this is done with his one system, one balloon.”
At the Transcatheter Therapeutics (TCT) conference in October, the company reported results from its first-in-human POWER PAD I study in nine patients.
Moderately to severely calcified superficial femoral and popliteal arteries were enrolled in this study. The average treated lesion length was 250 mm. Treatment with AVS's pulse endovascular lithotripsy system was successful in all nine patients, with no significant adverse events.
“We concluded this trial with great results in some patients who are difficult to treat,” Chisena said. ”[The study] It definitely helped solidify our faith in technology. Although it is simple to use, it has many benefits for patients and physicians who use it. ”
crowded field
Although AVS may eventually expand to other indications, including coronary, it is currently focused on peripheral disease as there is little unmet need. “Peripheral vessels are notoriously difficult to treat when calcium is present,” Chisena says. “Typically, these vessels have diffuse disease, the calcium is eccentric and difficult to access, and there is a large rebound when treated.”
Shockwave Medical has already demonstrated market potential for interventional solutions for calcified vascular lesions and is sure to attract more competitors. The company expects to record revenue of about $730 million this year, representing about 49% year-over-year growth. Shockwave's market capitalization is approximately $7.4 billion (see also “Shockwave acquires Neovasc, hopes angina-reducing device will complement coronary lithotripsy” – Medtech Insights, January 18, 2023) ).
AVS is confident that it can succeed in the market, even if it becomes increasingly crowded. “Even if there are products “just as good'' as Shockwave. [devices] It will be successful, but I won't go into too much detail, but AVS has a few things in store that will give doctors a lot of benefits in treating these vessels. ”
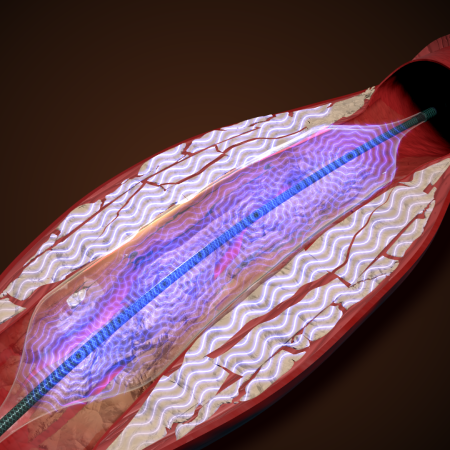
AVS Pulse Cracking Vascular Plaque
The company is led by Executive Chairman Mark Toland, an industry veteran who previously led Boston Scientific's US cardiovascular commercial team. He joins AVS through Biostar Capital, where he recently led AVS' $28.8 million Series B funding round with CUE Growth Partners.
Sean Gilligan, formerly vice president of program management and research and development at Boston Scientific, joined AVS as chief operating officer in April, and the company moved to new offices in Boston in September. .
After an eventful 2023, the company is now looking forward to the start of pivotal trials. “Since Series B, we've been building the company in parallel with development, and we're going to start testing around that soon, probably in the next six months or so,” Chisena said.
Use the player above or Medtech Insight Heart Conversation Listen to the full interview with Robert Chisena on your favorite podcast platform.
Editor's note: Chisena alluded to this in an interview. new york times Investigation of physicians marketing unnecessary and potentially harmful peripheral atherectomy procedures. Some investors had expected Shockwave's stock price to decline through most of the second half of 2023 as negative publicity would slow overall peripheral intervention growth.
”[Shockwave] “There are really sick patients who need this kind of treatment,” Chisena said.
Shockwave told analysts that these reports have had little impact on demand for its endovascular lithotripsy technology. “We don't see any changes to our procedures,” Shockwave CEO Douglas Godshall said in response to questions. Times” Presented at the Wells Fargo Healthcare Conference in September.
References
Shockwave launches first all-female study on coronary intervention
Abbott acquires CSI to add peripheral and coronary atherectomy technology
FDA Approves Shockwave Coronary Endovascular Lithotripsy System
hear more
The complete recording of this podcast, like all other Citeline podcasts, is available on Apple Podcasts, Google Podcasts, SoundCloud, TuneIn, Spotify Podcasts, and if one of these platforms is set as your default podcast provider Available via smart speaker. .

