This study demonstrated that the cosmetic products that were randomly selected for evaluation had significant amounts of hydroquinone, mercury, and arsenic. Table 2 lists the specific characteristics of the skin-lightening cosmetics that were the subject of this investigation, whereas Table 3 lists the optical characteristics of the calibration curve for the UV spectrophotometer. The spectra patterns for the reference hydroquinone powder at different concentrations, ranging from 10 to 50 µg/mL, are shown in Figure 1. Figure 2 displays the samples with hydroquinone concentrations that are above the permissible limit for cosmetic products. The precision for the analysis of hydroquinone is shown in Table 4. Table 5 shows the optical characteristics of mercury and arsenic as well as the AAS instrumentation parameters. While the levels of arsenic in samples F and H were extremely high, samples A, C, and D showed significant amounts of mercury (Hg) in the cosmetic samples, which are over the permitted limit of 1 µg/g for cosmetic products (Figure 3).
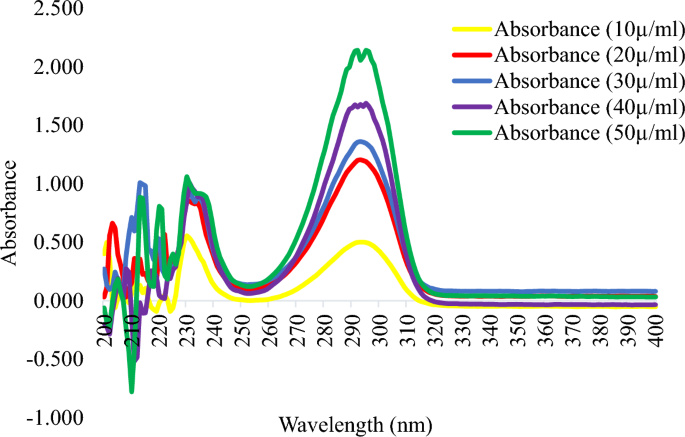
Spectra of standard hydroquinone powder between 200 and 400 nm for five different concentrations show maximum absorbance at 290 nm.
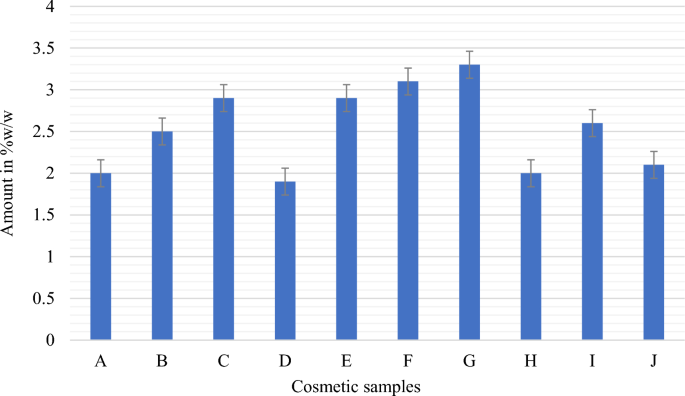
Percentage composition of hydroquinone in cosmetic samples A-J using an ultraviolet spectrophotometer at 290 nm.
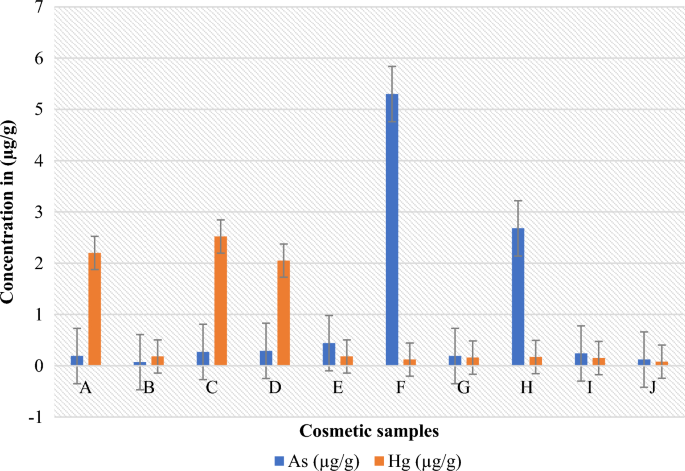
Mercury and arsenic levels in µg/gin skin-lightening cosmetics measured using AAS at wavelengths of 253.7 and 193.7 nm, respectively.
Descriptive characteristics of the skin-lightening cosmetic samples
All the products were within the labelled expiration date as of the time of the analysis, while only six of the ten products were registered with the National Agency for Food and Drug Administration and Control (NAFDAC). Most of the products evaluated were manufactured in Cote d’Ivoire (54.6%) and the Republic of Togo (27.3%). Two of the products investigated had no information about the country of origin. Three of the products explicitly indicated the presence of 2% hydroquinone on their product labels. Similarly, one of the cosmetics indicated the presence of hydroquinone on its label but omitted the amount.
In contrast, the remaining six products neither acknowledged the presence of hydroquinone nor disclosed any related amount on their skin-lightening cosmetic labels. Also, none of the ten skin-lightening cosmetics purchased provided any information about the presence of mercury or arsenic on their product labels. Furthermore, one sample out of the ten failed to include a batch number on its label. Surprisingly, it was observed that none of the ten skin-lightening cosmetics analysed were manufactured in Nigeria. This suggests that a significant portion of these skin-lightening products were imported into the country, potentially without adequate consideration for the possible harmful effects of their constituents.
Identification of reference hydroquinone powder using UV scans and melting points
From Figure 1, the lowest and highest absorbance readings were obtained at concentrations of 10 and 50 µg/mL, respectively. The peak absorbance was obtained at 290 nm, which corresponds to the British Pharmacopoeia (BP) specification for hydroquinone (BP 2016). The melting point obtained was 172 °C, which also corresponds to the BP specification. Thus, the identity of our reference hydroquinone was confirmed with a UV scan and melting point determination.
Identification of hydroquinone in the skin-lightening cosmetic samples
The result obtained shows that all ten skin-lightening cosmetic samples had comparable Rf values with the reference hydroquinone powder, indicating the presence of hydroquinone in the samples.
Quantitative determination of hydroquinone in skin-lightening cosmetic samples
The concentration of hydroquinone in each cosmetic sample was determined using a validated UV-spectrophotometric method at a wavelength of 290 nm. The method showed good linearity over a concentration range of 5 to 50 µg/mL (Table 3). The linearity equation was y = 0.0307x + 0.0192, and the mean regression coefficient (r2) was 0.9993 (Table 3). The method showed good precision and accuracy, with percentage relative standard deviation (%RSD) and percentage (%) recovery ranges of 0.01–0.35% and 95.85–103.56%, respectively (Table 3). The results showed that all the %RSD was below 2%, thereby attesting to the sensitivity, reliability, and accuracy of the analytical method. The limits of detection (LOD) and limits of quantification (LOQ) obtained were 0.7524 and 2.2801 µg/mL, respectively (Table 3). Intra-day validation gave the lowest and highest percentage recovery of 98.23% and 104.82%, respectively. The inter-day validation gave 98.23% and 99.85% as the lowest and highest percentage recovery, respectively (Table 4).
Quantitative analysis to determine the content of hydroquinone in the ten cosmetic samples showed varying amounts, ranging from 1.9 to 3.3% w/w. Samples D and G contained the lowest and highest amounts of hydroquinone, respectively. Further, samples A, D, and H contained 2%, 1.9%, and 2% w/w hydroquinone, respectively (Fig. 2), which were within the 2% w/w maximum permissible limit set by the WHO for cosmetics48. The remaining seven samples (B, C, E, F, G, I, and J) were found to contain hydroquinone in amounts ranging from 2.1 to 3.3% w/w, surpassing the allowable limit of 2% for cosmetic products48. In this study, most of the cosmetic packages did not provide information about the presence of hydroquinone, while only four samples explicitly stated the inclusion of hydroquinone on their product labels. Among these, only sample D contained the permitted amount of hydroquinone (1.9% w/w). Conversely, samples E and I contained 2.9 and 2.6% w/w of hydroquinone, respectively, exceeding the permitted threshold for cosmetics. Despite claims on their labels that they contained 2% w/w hydroquinone, samples D, E, and I were found to contain higher levels. Similarly, sample F was assayed to contain 3.1% w/w hydroquinone, surpassing the allowable limit. Notably, although sample F indicated the presence of hydroquinone on its label, it failed to specify the amount present.
These findings unveil a concerning reality: many skin-lightening cosmetics marketed in Ilorin, Nigeria contain varying and often excessive amounts of hydroquinone. What is particularly troubling is that these hydroquinone concentrations surpass the permissible limits for cosmetics. Consistent with other studies are the facts that most skin-lightening products contain chemicals such as hydroquinone and heavy metals that make them unsafe for use13,28. Despite this knowledge, these products continue to be widely distributed and used without strict regulatory control. Our findings also revealed that some of these cosmetic products marketed in Ilorin, Nigeria lack NAFDAC approval. Regrettably, many of those with NAFDAC approval failed quality assessments, surpassing the permissible limits for hydroquinone in cosmetics28,49. Prolonged and consistent use of hydroquinone-containing cosmetics elevates the risk of developing disorders attributed to hydroquinone4,25,26,27.
Long-term use of hydroquinone in cosmetics can lead to severe and significant adverse health effects, including conditions such as carcinoma4,9,24 and ochronosis21,25. These are diseases of public health importance, underscoring the necessity to proactively prevent their occurrence. Therefore, implementing preventive measures becomes paramount, and a crucial aspect of this involves eliminating potential risk factors such as harmful substances in cosmetic products. Moreover, the ramifications of long-term hydroquinone usage extend beyond these serious disorders. Prolonged exposure to hydroquinone has been associated with a range of adverse effects, including irritative dermatitis, melanocyte destruction, contact dermatitis, neuronal damage, and mutations4,21,26,27,28.
Mercury and arsenic contents in skin-lightening cosmetic samples
Skin-lightening cosmetics used by women, young girls, and certain men for cosmetic purposes often contain heavy metals like mercury and arsenic50. The present study evaluated the mercury content of ten samples of skin-lightening cosmetics. The observed mercury concentrations ranged from 0.08 to 2.52 μg/g, with the highest and lowest concentrations being detected in samples C and J, respectively (Fig. 3). In an effort to ensure consumer safety, both the FDA and WHO have set a limit of 1.0 μg/g for mercury content in skin-lightening cosmetics13,40,49,51. Significantly, the findings of this study revealed that each of the ten examined skin-lightening cosmetics contained varying amounts, and three of these samples exceeded the permissible limit set for cosmetic products, indicating an unsettling presence of elevated mercury levels.
Similarly, none of the cosmetic products displayed product labels indicating the presence of mercury on their packaging, corroborating the previously reported findings4. The exceedingly high Hg levels in some of these cosmetics raise serious health concerns40. The identification of excessive mercury levels in three of the examined cosmetics serves as a crucial warning of potential health risks for regular users of skin-lightening cosmetics. Additionally, it is important to note that even relatively small quantities of mercury in skin-lightening cosmetics have been shown to have harmful effects. For instance, it was discovered that mice exposed to skin-lightening cosmetics containing 0.319 μg/g of mercury had histological abnormalities in their brain, liver, and kidney tissue38.
Also, regular use of mercury-containing cosmetics during pregnancy and lactation was reported to cause growth abnormalities in newborns11,52. Further, long-term exposure to high concentrations of Hg has been associated with adverse health effects, including hepatic damage53, renal impairment54,55,56, and neurological damage11, such as ataxia, muscle weakness, increased tendon reflex, numb limbs, speech distortion, and difficulty chewing and swallowing11. Many other studies have also reported high mercury concentrations in skin-lightening cosmetics38,39. Prolonged exposure to mercury causes significant health risks of utmost public health importance. Such exposures have contributed to a heightened prevalence of cancers, neuronal disorders, and hepatic and renal failure11,13,36.
The severe health problems caused by exposure to Hg have prompted many countries, like the EU29, the US57, Canada58, and some African countries39(Ghana, Uganda, and Nigeria), to impose bans on the use of Hg in cosmetic products40,57. Nevertheless, the effects of the ban on mercury in cosmetics remain uncertain in Nigeria, given that cosmetics containing mercury are still widely consumed by users5. The intentional use of mercury in skin-lightening cosmetics is prohibited by the Drugs and Cosmetics Act59. The prevalent occurrence of unregulated hazardous chemicals and heavy metals in skin-lightening cosmetics is a well-documented issue in many developing countries. Nonetheless, this concern transcends national boundaries and is indeed a global problem. For example, Sahu et al. (2014)59 reported mercury in 17 of 32 skin-lightening cosmetics investigated. In a similar study by Hammann et al. (2014)60, a total of 549 skin-lightening cosmetics selected from 32 countries were confirmed to contain mercury. Addressing this menace requires the implementation of stringent regulations and effective compliance strategies to reduce the prevalence of cosmetics containing mercury. From a global perspective, it becomes imperative to establish legislation and rigorously enforce measures that prohibit the production, importation, and export of cosmetics containing mercury. Such measures would undoubtedly prove advantageous in mitigating the public health hazards associated with the routine use of mercury-containing cosmetic products.
Conversely, samples B and F contained 0.07 and 5.3 µg/g of arsenic, respectively, representing the lowest and highest measured amounts (Fig. 3). When compared to the previous studies, these values stand out as significantly elevated. For instance, the amount of arsenic found in cosmetics by Adepoju-Bello et al. (2012)61 ranged from 0.006 to 0.013 µg/g, and a similar study reported a maximum of 0.2 μg/g arsenic in body lotions62. However, the amounts of arsenic in samples F and H of our study were 5.3 μg/g and 2.68 μg/g, respectively (Fig. 3). These amounts were too high in cosmetic products, although some previous studies also reported high amounts of arsenic in skin-lightening cosmetics. For example, Mohammed et al. (2017)18 reported a range of 1.016–6.612 μg/g of arsenic in skin-lightening cosmetics, while Alquadami et al. (2013)63 reported arsenic levels between 0.34 and 14.76 μg/g in 34 examined skin-lightening cosmetics.
It is noteworthy that despite our findings of elevated arsenic levels in samples F and H (Fig. 3), the product labels on the cosmetic packaging did not mention arsenic as one of the ingredients. Furthermore, our study identified arsenic in all ten skin-lightening cosmetics studied. This raises questions regarding the source of arsenic in cosmetics, which could stem from intentional inclusion or inadvertent contamination during the manufacturing process. The introduction of arsenic into cosmetics could potentially occur through the tear and wear of metallic equipment and machinery used during the manufacturing processes64. However, it is important to note that significant arsenic contamination through these processes is not typically anticipated to be substantial.
Chronic exposure to arsenic has been reported as a significant risk factor for developing various types of cancer11,65,66,67. Long-term exposure to arsenic has been associated with a range of health problems. These encompass palmar and solar keratosis, gastrointestinal symptoms such as vomiting and diarrhea, dermatological abnormalities, neuropathy, ischemic heart disease, cognitive impairment including confusion and memory loss, respiratory disorders, hormonal imbalance, compromised immune system function, and an increased risk of diabetes64,66,67. The ability of arsenic to induce oxidative stress and mitochondrial dysfunction is the main cause of the observed neurological diseases11. Oxidative stress damages the DNA, which results in neuronal cell death14. Despite the harmful effects of arsenic, it has been found to be a component of eye shadow, lotions, cosmetics, and lipsticks68. Regrettably, no strict regulations prohibit the use of arsenic in cosmetic preparations except in the European Union69. Hence, there is currently no established limit to the amounts of arsenic that are permitted in cosmetics. This regulatory gap underscores the urgent need for comprehensive guidelines to restrict and monitor the presence of arsenic in cosmetic preparations to mitigate potential health hazards associated with its usage.
The health hazards and public health relevance of undue exposure to hydroquinone, mercury, and arsenic require that policymakers and regulatory authorities put strict restrictions on the production and importation of skin-lightening cosmetics containing these dangerous chemicals. Such measures are crucial in curbing the proliferation of unregulated cosmetic products in the market, thereby ensuring the well-being of Nigerian citizens, and mitigating the economic hardship brought about by the burden of associated health problems.

